Friday 31 May 2013
Wednesday 29 May 2013
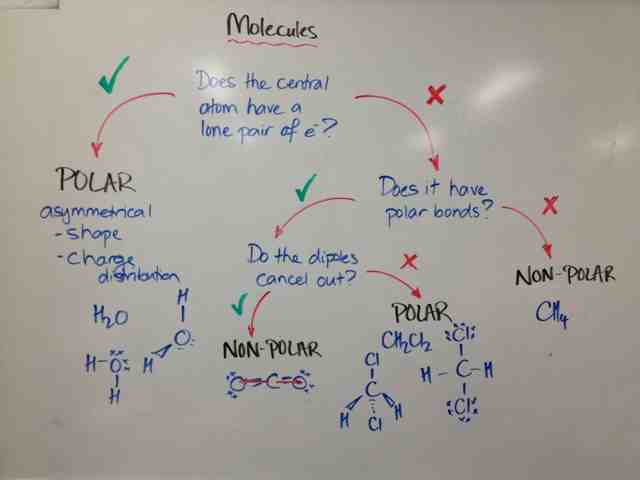
Shapes of Molecules
The shapes of molecules are the last clue for helping determine molecular polarity:
Tuesday 28 May 2013
Lewis Diagrams
Lewis Diagrams and Structures are very useful for helping us predict molecular polarity.
Monday 27 May 2013
Wednesday 22 May 2013
Substance Type Overview
It is very important to understand that "Matt" is not an attractive force, so please do not use it as an answer in assessments.
Tuesday 21 May 2013
Chart - Type of Solid, Type of Particle, Attractive Forces Between Particles
http://chemicalminds.wikispaces.com/file/view/solidsANSWERS.pdf
This is a fully answered chart for multiple types of solids, same type of question from one of the question sheets from today.
This is a fully answered chart for multiple types of solids, same type of question from one of the question sheets from today.
Monday 20 May 2013
Molecular Solids
 Molecular Solids have some characteristic properties:
Molecular Solids have some characteristic properties:- low m.p./b.p.
- electrical and thermal insulators
- often brittle
These properties need to be explained in terms of the forces between the molecules (particles). These forces are called van der Waal's forces. Before we can understand these, we need to understand molecular polarity, which requires us to understand intra-molecular bonding (covalent), shapes and electron cloud size.
Tuesday 14 May 2013
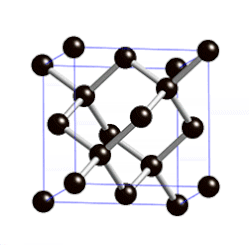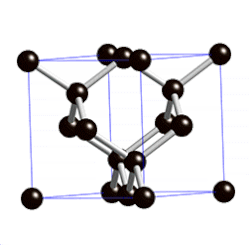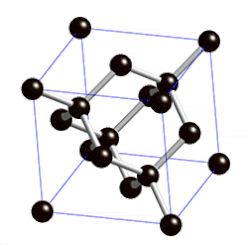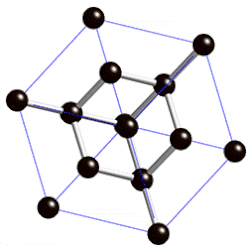
Covalent Networks
Monday 13 May 2013
Metallic Solids
Most elements are metals. Metals have some characteristic properties which can be explained by the metallic bond and the structure of metals in their solid state:
- Electrical Conductivity
- Thermal Conductivity
- Malleability/Ductility
- Relatively High m.p (except Hg)
Wednesday 8 May 2013
Subscribe to:
Posts (Atom)